NovaScan
NovaScan
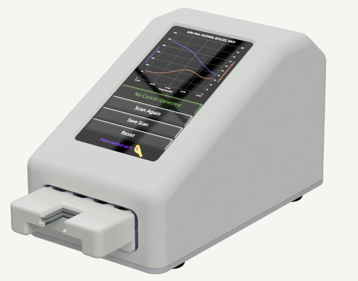
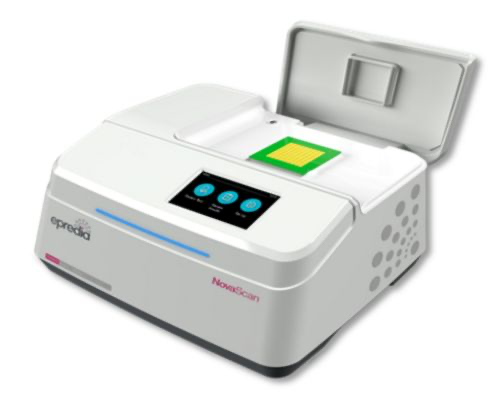
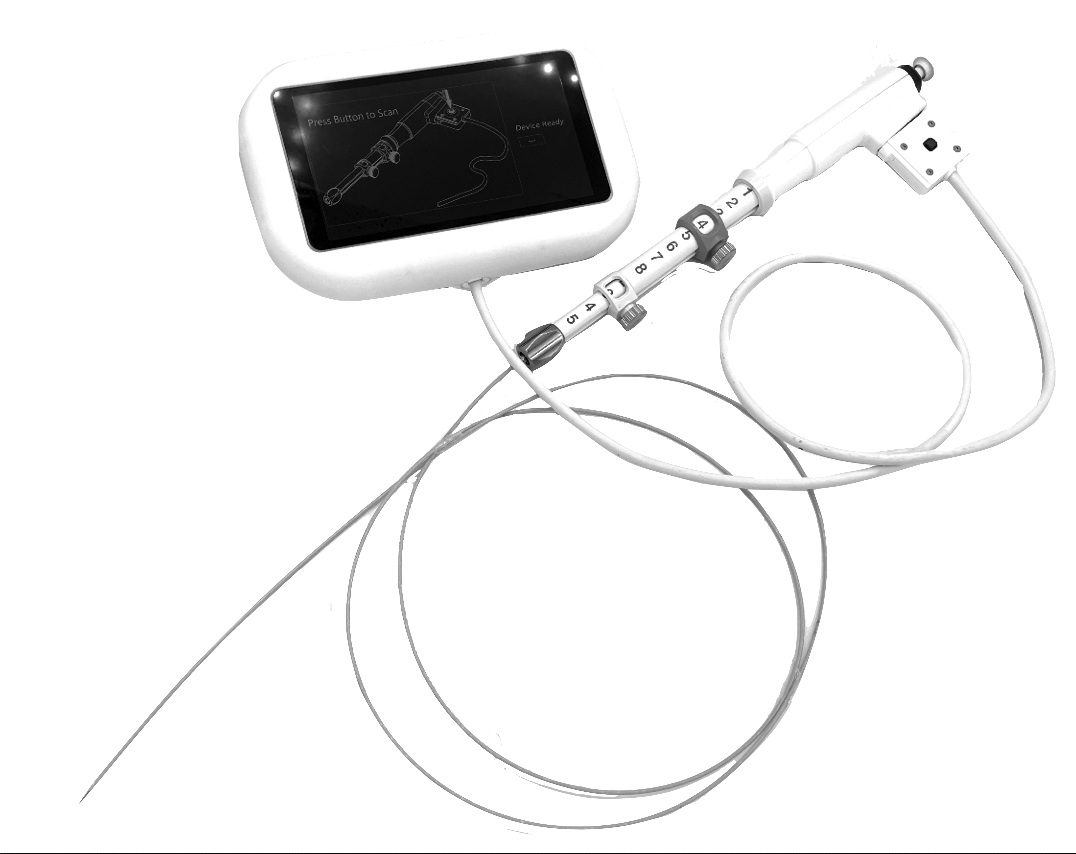
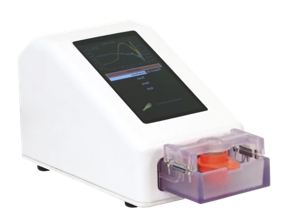
AMDD was brought into NovaScan to commercialize devices showing promising data in the lab. Provided staff augmentation, served as the Chief Technology Officer and VP of Product Development. Built diverse team of engineering scientists and data scientists. Provided Structured engineering development, clinical trials, intellectual property development, data analytics and team development/ leadership/ management. Strategic product roadmap development – engineered three products in multiple market segments Ran multiple multi-site human clinical trials, multiple animal trials. Engineered an AI based algorithm development and regulatory strategy for productization. Regulatory and clinical strategy development, FDA pre-sub meetings. Strengthened patent portfolio, filed over seventeen patents and authored multiple journal articles.
Applied Services
- Systems Engineering
- Compliance Engineering
- Electronics Design
- Animal Testing
- Physician Training
- Risk Assessment
- Business Development
- IP management
- Staff Development
- Clinical Trials
- FDA PreSub